




Home Oncology



The EntroGen NGS Targeted Hotspot Panel is a comprehensive assay that detects clinically relevant mutations in solid tumors using next-generation sequencing, compatible with fresh frozen and FFPE samples. It offers high sensitivity, low DNA input, and the ability to batch up to 12 samples in a single run, making it ideal for labs with limited sample volumes.



EntroGen’s BRCA Complete™ Expanded Panel is a comprehensive NGS solution that detects both germline and somatic mutations in genes like BRCA1, BRCA2, CHEK2, PALB2, RAD51C, and TP53 with high sensitivity and specificity. Compatible with blood, fresh frozen, and FFPE samples, the panel reduces allele dropouts and off-target reads using a tiled amplicon PCR approach. It includes reagents for library preparation and user-friendly software for easy mutation identification, along with quality control assays to ensure high-quality data without repeat sequencing.


This is a precision diagnosis panel to detect major genetic variations related to malignant tumors in blood using NGS technology.


The new 21 CFR Part 11 Module for the 7500 Real-Time PCR System offers the flexibility of user-customizable CFR configuration settings to assist in compliance with the Code of Federal Regulations Title 21 Part 11.


The Applied Biosystems QuantStudio 6 Pro Real-Time PCR System adds innovative smart features to the real-time PCR (qPCR) workflow, all in a compact footprint. With facial authentication, voice commands, Smart Help, multiplex capability, motorized interchangeable blocks, simplified workflow, intuitive software, and large capacitive touch-screen interface, the QuantStudio 6 Pro system offers exceptional reproducibility with minimal well-to-well and instrument-to-instrument variation. Designed for ultimate ease-of-use, the system offers high quality, excellent reliability, and an optimal user experience for researchers who want to work smarter in the lab.
This package includes the QuantStudio 6 Pro Real-Time PCR System, SmartSmart Orientation, and one-year extended warranty.
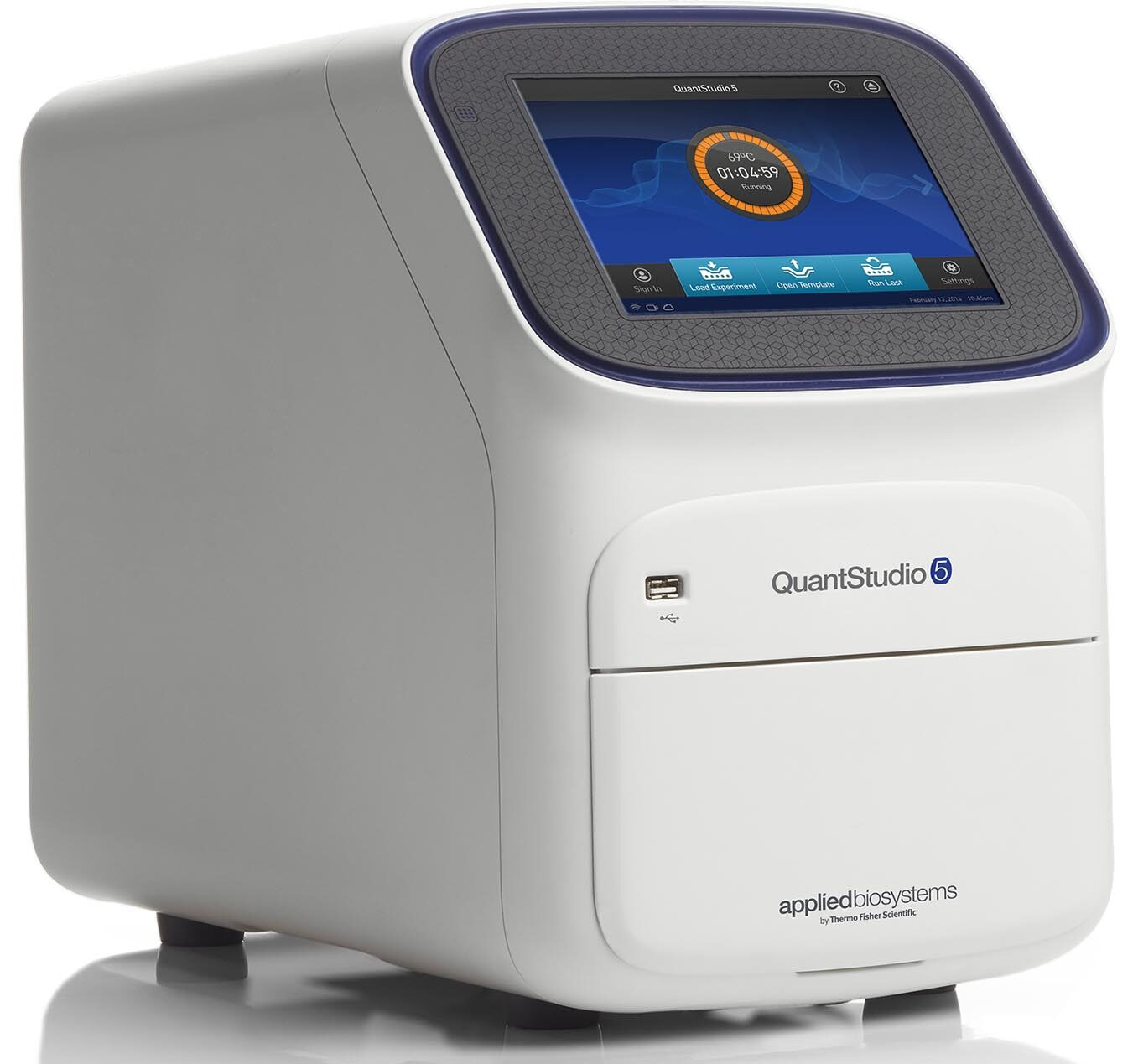

The Applied Biosystems QuantStudio 5 Real-Time PCR System is designed for users who need superior performance, maximum dye versatility, and security options in a real-time PCR system that is affordable and easy to use. The optimized Design and Analysis software is ideal for both first-time and experienced users. When connected to iConnect, Thermo Fisher’s cloud-based platform, the QuantStudio 5 system provides access to your data wherever and whenever you want. Using proven OptiFlex technology (featuring six decoupled channels and white LED) and featuring six independent Veriflex temperature zones, the QuantStudio 5 96-well system enables improved data accuracy and sensitivity for a broad range of genomic applications. The QuantStudio 5 system also offers the built-in software features of electronic record security and prevention of unauthorized instrument access to assist with 21 CFR Part 11 compliance.
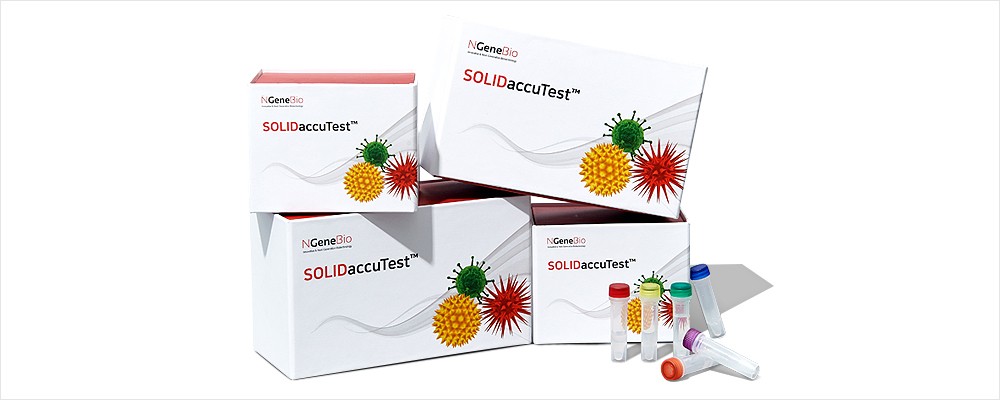


SOLIDaccuTest™ is an excellent tool for discovering variants associated with solid tumors using a comprehensive approach to next-generation sequencing (NGS). It reflects the latest research trends and is optimized for medical purposes by selecting essential genes of solid tumors including lung, colon, breast, skin brain, stomach and ovarian cancers, etc.



SOLIDaccuTest™ is an excellent tool to explorer variants associated with solid tumors using a comprehensive method of next-generation sequencing (NGS). It reflects the latest research trends and is optimized for the medical purpose by selecting essential genes of solid tumors including lung, colon, breast, skin brain, gastric, ovarian cancers, etc.
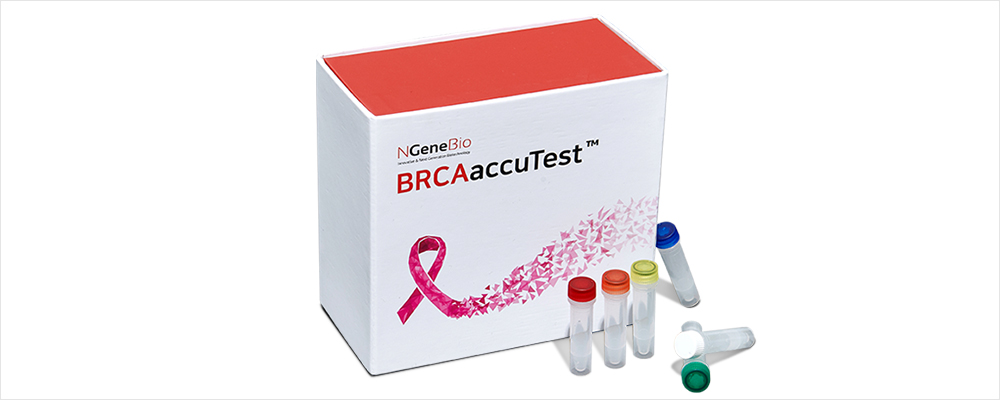


BRCAaccuTest™ PLUS with CE-IVD certificate for HBOC Breast/Ovarian Cancer Test



BRCAaccuTest™ & BRCAaccuTest™PLUS is a regent for producing libraries for
analyzing the BRCA ½ genes using the NGS (next-generation sequencing) method,
which analyzes genomic DNA derived from blood or FFPE tissues.


Model: NovaSeq 6000
Manufacturer: Illumina/USA
– Scalable platform
Match data output, time to results, and price per sample to study needs
– Flexible performance
Configure sequencing method, flow cell type, and read length to support a broad range of applications
– Streamlined operation
Increase lab efficiency with a simplified workflow and reduced hands-on time


The NextSeq 2000 Sequencing System uses patterned flow cells similar to those that power the NovaSeq™ 6000 System. The result is a highly flexible and scalable benchtop system that offers the highest cluster density flow cell of any on-market NGS system to date, driving down the cost per gigabase (Gb) of the sequencing run.


Model: NextSeq 1000
Manufacturer: Illumina
Origin: Singapore/United States
Launching year: 01/2020
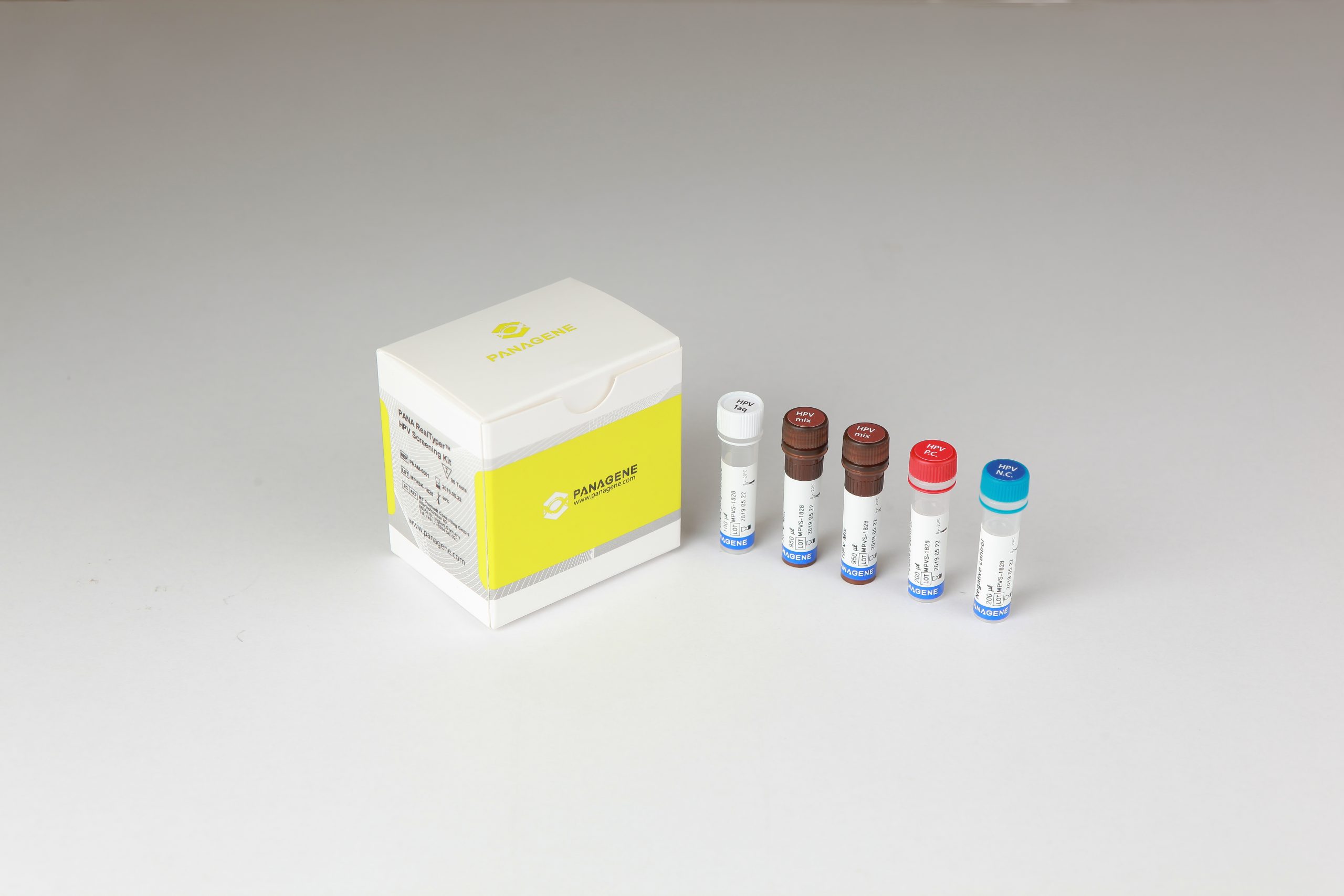

The PANA RealTyper™ HPV Screening Kit is a CE marked diagnostic device in accordance with the European Union in vitro Diagnostic Medical Device Directive 98/79/EC

PANA RealTyperTM HPV Kit is a CE marked diagnostic device in accordance with the European Union in vitro Diagnostic Medical Device Directive 98/79/EC.


DxFLEX is a new clinical flow cytometry platform derived from the successful CytoFLEX. The advanced sensitivity and intuitive software DxFLEX makes flow cytometry routine for both novice and expert flow cytometry technicians and promotes standardization. Functionality in the autoloader facilitates accurate results and sample tracking.


Model: CytoFLEX
Manufacturer: Beckman Coulter Life Sciences/ USA
The CytoFLEX Flow Cytometer is the most innovative flow cytometer system of Beckman Coulter. With compact design and high efficiency in work, CytoFlex provides and easy to use system with the impressive performance you need. Our system allows the researchers just focus on the science with no worry about instrument. Its superior sensitivity and resolution throughout all configurations give it the edge over other cytometry systems four times its size.
– Up to 3 lasers Violet-Blue-Red (V-B-R) and fully activated with 13 colors
– Ability to measure 100 nm particles with violet laser
– Includes 13 band pass filters: 450/45, 525/40 (2), 585/42, 610/20 (2), 660/10 (2), 690/50, 712/25, 780/60 (3)
– For higher throughput applications an optional plate loader module is available
– Smart software in acquisition and analysis CytExpert


Model: NovaSeq 6000
Manufacturer: Illumina/USA
– Scalable platform
Match data output, time to results, and price per sample to study needs
– Flexible performance
Configure sequencing method, flow cell type, and read length to support a broad range of applications
– Streamlined operation
Increase lab efficiency with a simplified workflow and reduced hands-on time


Model: iSeq 100
Manufacturer: Illumina/USA
Illumina’s smallest next-generation DNA sequencing system, with small, fast and efficient sequencing throughput, suitable for all labs
– Fast data generation: Suitable for small projects, on a dedicated device, low throughput with fast turnaround times
– Convenient operation: Control the sequencing process from start to finish and ensure independent sequencing instead of outsourcing.

TruSight Tumor 26 Kit includes library preparation reagent set and uses next-generation sequencing (NGS) technology to provide a comprehensive assessment of 26 genes that are commonly related to mutations in solid tumors and somatic variants


Developed with cancer genomics experts in collaboration, oligos ready-to-use, pre-engineered oligos allow researchers to sequence a wide range of genes and single nucleotide polymorphisms (SNPs) in advance. this is related to cancer predisposition

TruSight Tumor 26 Kit include reagents to prepare for Library preparation samples, sequencing 26 genes relating to solid cancer, variant of soma in NGS – Next Generation Sequencing of Illumina


Model: Nextseq 550
Manufacturer: Illumina/USA
– Is the only sequencing system capable of deploying applications running sequencing and reading microarray biochips
– New generation DNA sequencing system using synthetic sequencing technology: Sequencing by Synthesis – SBS
– The whole process of gene sequencing is conducted on the surface of flow cells, convenient to use and easy to manipulate.


Model: MiSeq
Manufacturer: Illumina/USA
– The MiSeq System combines cluster generation, amplification, sequencing and data analysis on a single system
– The system is most widely used, with next-generation sequencing technology – Sequencing by Synthesis – SBS


Model: MiniSeq
Manufacturer: Illumina/USA
– New generation DNA sequencing system using synthetic sequencing technology: Sequencing by Synthesis – SBS
– Next Generation Sequencing, allowing to run the entire DNA and RNA amplification process combined with sequencing analysis on a single system
– Maximum reading capacity: 7.5 Gb (equivalent to 7.5 billion bases) within 24 hours
– Data Quality (Quality Score) > Q30 (accuracy 99.9%)

Code: PNAC-3002
Packing: 25 tests/set
Certificate: CE-IVD

Code: PNAC-3002
Packing: 25 tests/set
Certificate: CE-IVD

Code: PNAR-1006
Packing: 25 Pounds/set
Certificate: CE-IVD

Code: PNAC-1101 (43 mutations)
Packing: 25 tests/set
Certificate: CE-IVD