GeneProof Hepatitis C Virus (HCV) PCR Kit uses PCR technology to detect DNA, RNA of virus HCV present in patient samples.
CE-IVD Quality Certificate for Medical Diagnostic Use in vitro


GeneProof
Reagents
GeneProof Hepatitis C Virus (HCV) PCR Kit uses PCR technology to detect DNA, RNA of virus HCV
GeneProof Hepatitis C Virus (HCV) PCR Kit uses PCR technology to detect HCV virus DNA present in patient samples.
CE-IVD Quality Certificate for Medical Diagnostic Use in vitro
GeneProof Hepatitis C Virus (HCV) PCR Kit uses PCR technology to detect DNA, RNA of virus HCV present in patient samples.
CE-IVD Quality Certificate for Medical Diagnostic Use in vitro
Detection target: HCV gennotype 1-8
Detection threshold: 7.95 IU/ml, with 95% probability
Based on viral RNA amplification reaction by Reverse Transcriptase PCR with primers designed specifically for HCV RNA sequence, using reduced “hot start” technology maximum non-specific binding, resulting in high accuracy test results.

GeneProof

|
Name of Products |
Code |
Package |
|
GeneProof Hepatitis C Virus (HCV) PCR Kit |
HCV/ISEX/025 HCV/ISEX/050 HCV/ISEX/100 |
25 reactions 50 reactions 100 reactions |


The Individual Fecal Collection Kit is a conveniently pouched kit that includes all the components needed for collecting and stabilizing fecal samples. This kit includes a Fecal Collection Tube, a Feces Catcher, a Biohazard Bag, Gloves, and multi-language instructions. Featuring DNA/RNA Shield technology, samples collected with the Fecal Collection Tube are accurately and safely preserved for downstream analysis.
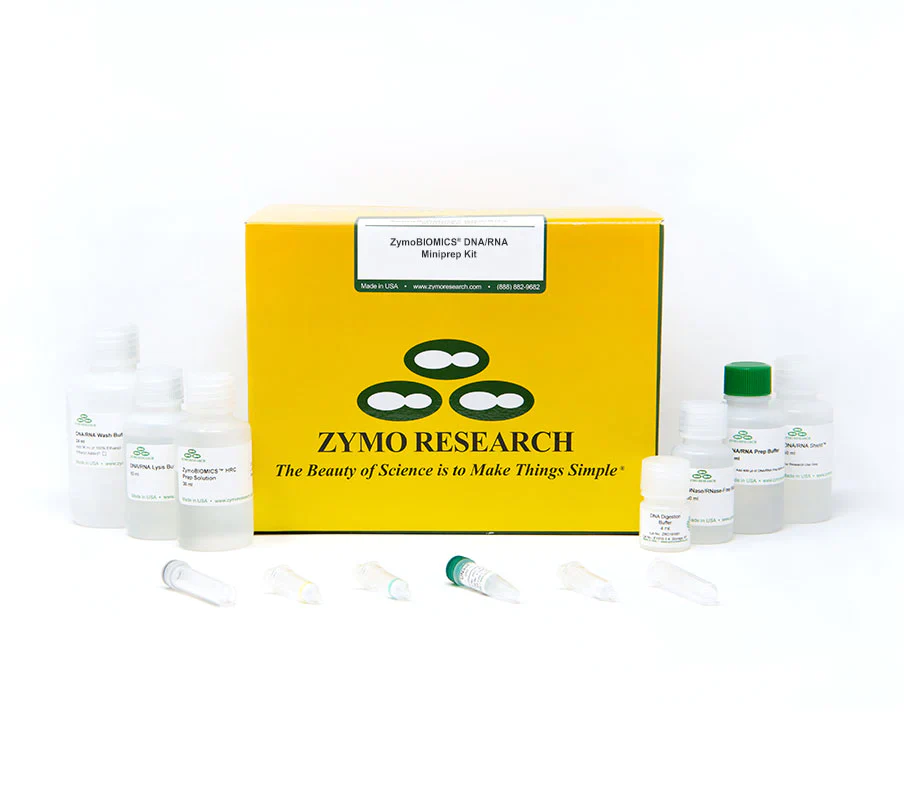

The ZymoBIOMICS DNA/RNA Miniprep Kit is designed for purifying DNA and RNA from a wide array of sample inputs (e.g. feces, soil, plant, water, and biofilms) that is ready for microbiome or metagenome analyses. The ZymoBIOMICS innovative lysis system eliminates bias associated with unequal lysis efficiencies of different organisms (e.g. Gram-negative/positive bacteria, fungus, protozoans, and algae). The provided DNA/RNA Shield preserves nucleic acids at ambient temperatures, providing an unbiased molecular snapshot of the sample. The procedure uses Zymo-Spin Column technology that results in high-quality DNA and total RNA (including small RNAs 17-200 nt) that is free of PCR inhibitors (e.g. polyphenols, humic acids, and fulvic acids). Ready for RT-PCR, arrays, sequencing, etc.


The ZymoBIOMICS Fecal Reference with TruMatrix™ Technology is a microbial reference material composed of stool from healthy donors for quality control, process validation, assay development, and proficiency testing. The fecal reference was homogenized in one large batch to produce consistency in every vial, eliminating typical lot-to-lot variability. With over 2 million preps available, this reference is intended to be used as a control for workflow performance, as well as comparing workflow consistency across replicates, studies, and laboratories. To facilitate methods comparison, a platform is in development to enable users to compare characterization data against applied methodology.
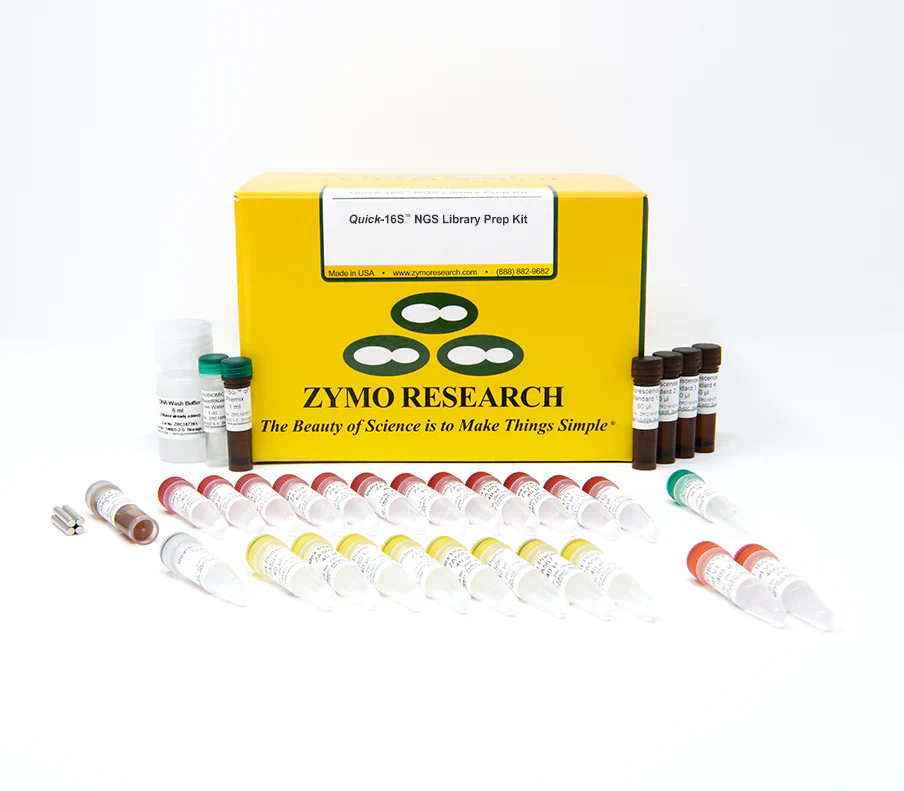

16S rRNA sequencing is a routine technique for microbiome composition profiling. Compared to shotgun metagenomics sequencing, 16S rRNA sequencing is more cost-effective and more robust; it generally requires less input DNA and is less impacted by the presence of host DNA. However, 16S rRNA sequencing has its own challenges. One major challenge is the formation of PCR chimeric sequences, which are artificial sequences resulting from the recombination of two or more PCR templates. Additionally, with common 16S primers, it is difficult to achieve both species-level resolution and broad phylogenetic coverage. Moreover, common 16S library preparation protocols used in the field have not been optimized to be cost-effective for large-scale applications. The Quick-16S NGS Library Prep Kit aims to standardize the library preparation process for 16S rRNA sequencing. Distinguishing features of the kit are described below. Fastest 16S rRNA Library Prep. The Quick-16S NGS Library Prep Kit utilizes real-time (quantitative) PCR (qPCR) rather than endpoint PCR for 16S rRNA amplification, enabling direct quantification of PCR products and eliminating the need for additional library quantification analysis such as TapeStation analysis or gel electrophoresis. An enzymatic clean-up is introduced between the two PCR steps, saving time and reducing costs as compared to lengthy AMPure bead-based clean-ups. With these features, the kit dramatically reduces the hands-on time of 16S library preparation. Simple. The Quick-16S NGS Library Prep Kit includes all the reagents needed to convert 96 DNA samples to a 16S library. The resulting library is directly compatible with the Illumina MiSeq without needing additional custom sequencing primers. Accurate. The utilization of real-time PCR also enables users to control PCR cycles. This limits chimera formation and PCR bias while obtaining enough products for subsequent sequencing. In most cases, the abundance of PCR chimeric sequences is maintained below 2%. Increased Coverage. Due to the rapid expansion of 16S rRNA databases, the insufficient microbial coverage of common 16S primer sets has become evident. Zymo Research has re-designed two common primer sets targeting the 16S V1-V2 and 16S V3-V4 regions based on the most updated 16S reference database and significantly improved their coverage.
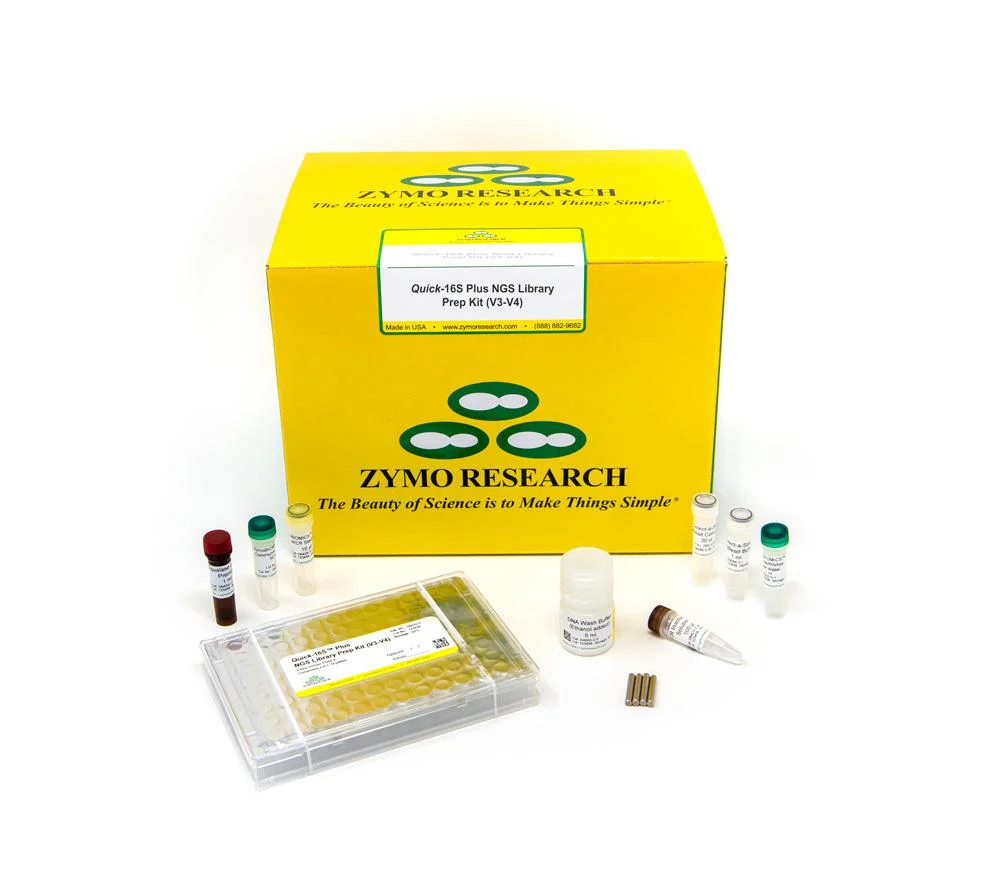

The Quick-16S Plus NGS Library Prep Kit (V3-V4, UDI) is the fastest and simplest NGS library prep targeting the V3-V4 region of the 16S rRNA gene for high-throughput sequencing. The automation-friendly protocol utilizes a single qPCR/PCR for combined targeted amplification and barcode addition using specially designed primers. After pooling by equal volume, a single clean-up of the final library is performed, rather than massive AMPure® bead-based clean-ups. Additional library quantification analysis such as TapeStation® analysis or gel electrophoresis are not necessary. With these features, the workflow dramatically reduces the hands-on time of library preparation to only 30 minutes.


The Quick-DNA Kits are ideal DNA isolation kits for easy, rapid isolation of total DNA (e.g., genomic, mitochondrial, viral) from a variety of biological sample sources. Whole blood (fresh or stored), buffy coat, buccal cells, cells from culture, saliva, and other biological liquid samples can be processed with these kits. Zymo-Spin Column/Plate technology enables high-quality DNA purification in minutes. PCR inhibitors are effectively removed, and the eluted DNA is ideal for PCR, nucleotide blotting, DNA sequencing, restriction endonuclease digestion, bisulfite conversion/methylation analysis, and other downstream applications.



The kit enables detection of Adenovirus respiratory species A–D, G and gastrointestinal species E-F


Certificate: ROU
Supplier: Illumina
For Mid and Low-throughput laboratories performing COVID-19 surveillance and identification of new variants


As a product of Panagene (Korea), the PANAMAX ™ 48 automatic extraction device works on the principle of using lysis and binding of samples by magnetic particles. Along with 3 samples kit ready-for-use for the process of extracting DNA/RNA/ccfDNA, this is the solution automatically 100%, just adding the sample to the correct specified wells, the […]