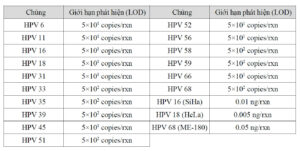
The PANA RealTyper™ HPV Screening Kit is an in vitro diagnostic reagent for detecting of human papilloma virus (HPV) from common specimen type, cervical swab and liquid based cytology (LBC) sample. This kit is an amplified DNA test for the qualitative detection of a total of 14 high-risk (HR) HPV types and 2 low-risk (LR) HPV types in a real-time PCR (polymerase chain reaction) system. This kit specially identifies 2 HR types such as 16 and 18 and 2 LR types such as 6 and 11 while concurrently detecting the other HR HPV types that includes 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68.