▶ Application of the kit
Detection ability: Detecting 43 mutations on codons 2, 3, 4 of the NRAS gene
High sensitivity and specificity even for small amounts of DNA (detects 2% mutation concentration with 10ng DNA sample)


Panagene
Reagents
Detecting 43 mutations on codons 2, 3, 4 of the NRAS gene
Code: PNAC-1101 (43 mutations)
Packing: 25 tests/set
Certificate: CE-IVD
▶ Application of the kit
Detection ability: Detecting 43 mutations on codons 2, 3, 4 of the NRAS gene
High sensitivity and specificity even for small amounts of DNA (detects 2% mutation concentration with 10ng DNA sample)
▶ Technology used:
Based on PNA-mediated Real-time PCR clamping technology using optimally designed PNA probes that adhere to the wild-type DNA template (DNA does not contain mutations).
▶ Input sample source: surgical tissue, FFPE sample
▶ Compatible systems: Biorad cfx 96, Light cycler 480, ABI 7500/7900, StepOnePlus, Rotor-Gene Q
▶ Certificate: CE-IVD Certified for Medical Diagnostic Use
Supplier
Panagene


The EntroGen NGS Targeted Hotspot Panel is a comprehensive assay that detects clinically relevant mutations in solid tumors using next-generation sequencing, compatible with fresh frozen and FFPE samples. It offers high sensitivity, low DNA input, and the ability to batch up to 12 samples in a single run, making it ideal for labs with limited sample volumes.
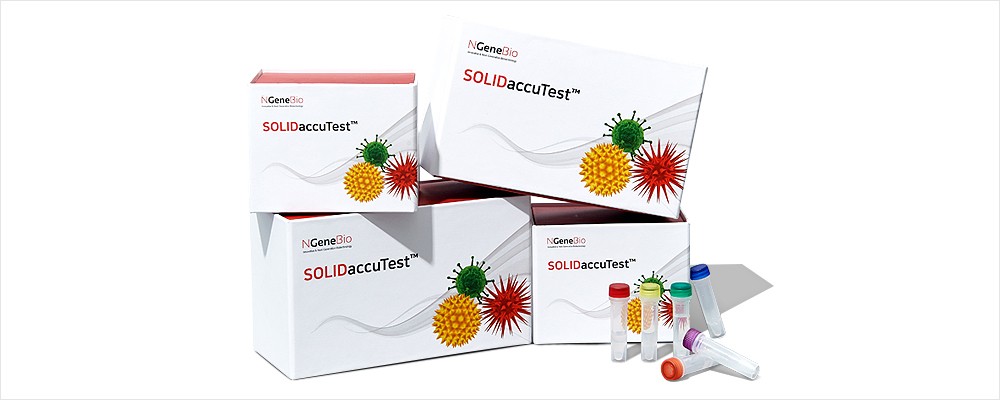


SOLIDaccuTest™ is an excellent tool for discovering variants associated with solid tumors using a comprehensive approach to next-generation sequencing (NGS). It reflects the latest research trends and is optimized for medical purposes by selecting essential genes of solid tumors including lung, colon, breast, skin brain, stomach and ovarian cancers, etc.



SOLIDaccuTest™ is an excellent tool to explorer variants associated with solid tumors using a comprehensive method of next-generation sequencing (NGS). It reflects the latest research trends and is optimized for the medical purpose by selecting essential genes of solid tumors including lung, colon, breast, skin brain, gastric, ovarian cancers, etc.
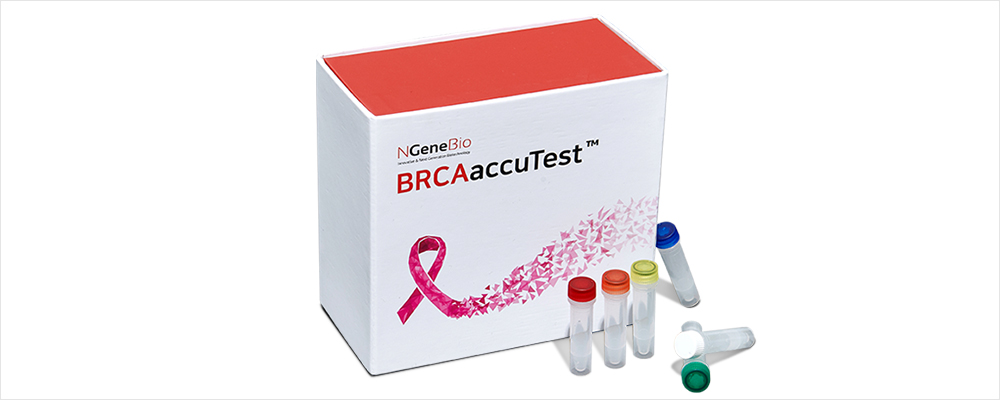

BRCAaccuTest™ & BRCAaccuTest™PLUS is a regent for producing libraries for
analyzing the BRCA ½ genes using the NGS (next-generation sequencing) method,
which analyzes genomic DNA derived from blood or FFPE tissues.


The NextSeq 2000 Sequencing System uses patterned flow cells similar to those that power the NovaSeq™ 6000 System. The result is a highly flexible and scalable benchtop system that offers the highest cluster density flow cell of any on-market NGS system to date, driving down the cost per gigabase (Gb) of the sequencing run.


Model: NextSeq 1000
Manufacturer: Illumina
Origin: Singapore/United States
Launching year: 01/2020
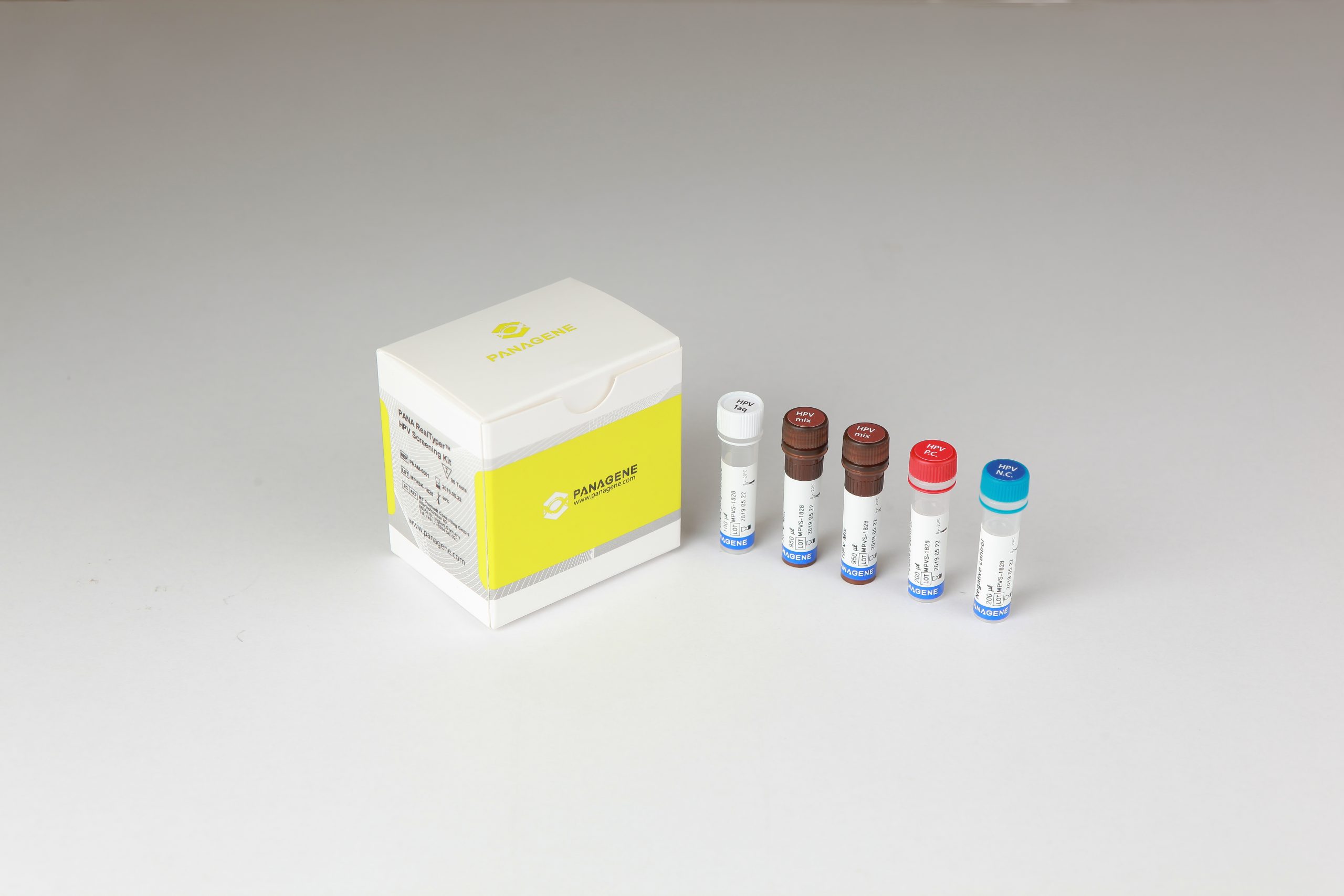

The PANA RealTyper™ HPV Screening Kit is a CE marked diagnostic device in accordance with the European Union in vitro Diagnostic Medical Device Directive 98/79/EC
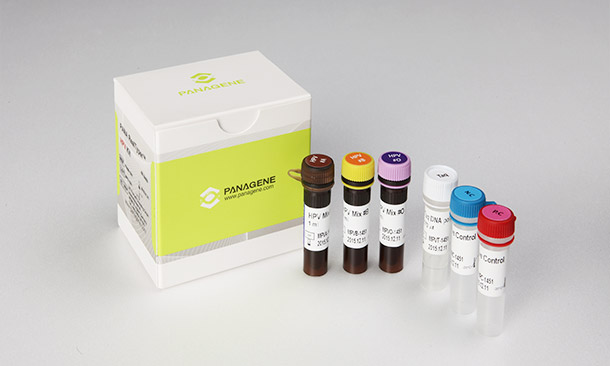

PANA RealTyperTM HPV Kit is a CE marked diagnostic device in accordance with the European Union in vitro Diagnostic Medical Device Directive 98/79/EC.


Model: NovaSeq 6000
Manufacturer: Illumina/USA
– Scalable platform
Match data output, time to results, and price per sample to study needs
– Flexible performance
Configure sequencing method, flow cell type, and read length to support a broad range of applications
– Streamlined operation
Increase lab efficiency with a simplified workflow and reduced hands-on time

TruSight Tumor 26 Kit includes library preparation reagent set and uses next-generation sequencing (NGS) technology to provide a comprehensive assessment of 26 genes that are commonly related to mutations in solid tumors and somatic variants